VISION-DMD investigated using quantitative Magnetic Resonance Imaging (MRI) to measure the difference in muscle involvement in the Phase 2b study.
MRI is a technique able to produce pictures from inside the body. It is a completely painless and harmless technique. The child lies on a bed and their legs are moved to the centre of the tunnel. Their head remains near the scanner opening and it is possible for a parent or carer to stay in the room near them for reassurance.
The child lies on the bed and enters the scanner feet first but their head stays out side the scanner bore. They are looked after throughout by a friendly radiographer! They are able to listen to stories or music during their scan. But be sure to keep nice and still!
Using MRI we can find out about the condition of the skeletal muscle in boys with Duchenne muscular dystrophy, and we can measure how it changes over time. In particular, we can find out how whether novel therapies can decrease the inflammation that is naturally present in the muscles of young boys with DMD as a measure of the acute disease process. Over time, the healthy muscle tissue is permanently replaced by fatty tissue and special MRI techniques are capable of measuring this very sensitively. By using MRI several times, we can estimate the effect that a therapy is having an effect on the underlying destructive processes.
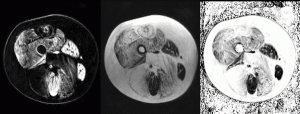
MRI is capable of distinguishing between the muscle tissue in the thigh that remains (left) and that which has been replaced by fat (middle) in Duchenne muscular dystrophy. From this information, MRI is capable of accurately measuring the percentage of muscle that has been replaced, and the change can be measured over time.
There have been a number of academic studies and trials which have used MRI as an endpoint. Sometimes the scanning sessions have lasted as much as an hour to collect comprehensive skeletal muscle data. Some boys find it difficult to keep still for this long. In this project, we are using our previous experience of DMD to present a simplified protocol which only requires 10 minutes of scanning (so the time in the scanner should be about 15-20 minutes in the scanner for boys who lie nice and still!). Cutting down the patient burden is crucial for making the regular use of MRI a practical reality in DMD trials carried out in multiple centres.
