
Overlapping Terms for Country-Specific Regulations
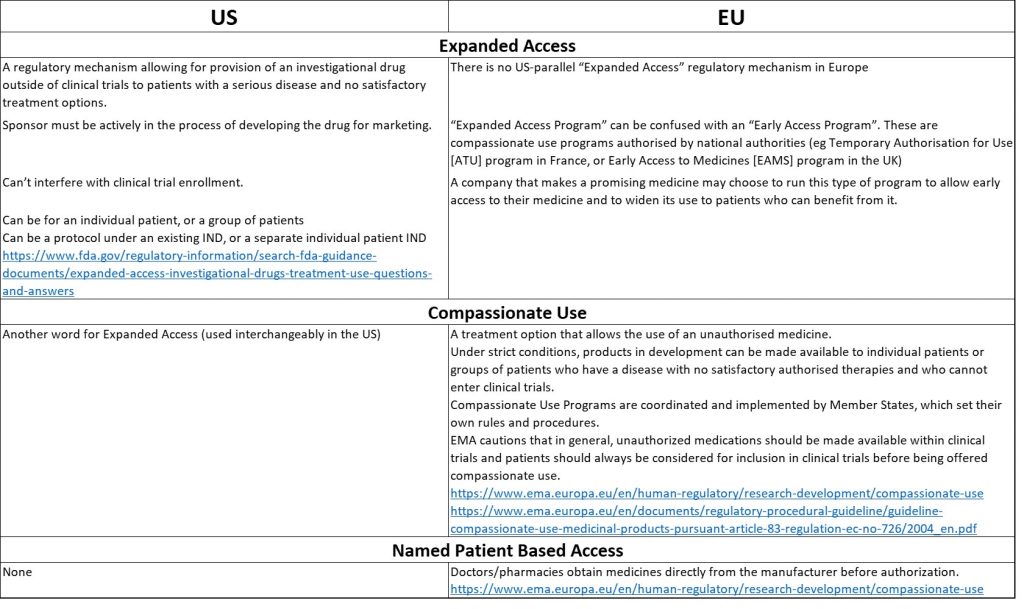
Describing programs is challenging, because similar terms convey different meanings in the US and EU. In the US, vamorolone is provided under an Expanded Access protocol. Under FDA regulations, expanded access is the means by which manufacturers of drugs may make investigational drugs available for physicians to treat a patient (s) with a serious disease or condition who cannot participate in a controlled clinical trial. In the case of vamorolone, there are no further extension trials in which the patients might enroll, so a VBP15-EAP protocol was submitted to the FDA. FDA guidelines include a requirement that drugs cannot expose patients to unreasonable risks given the severity of the disease to be treated, and that there are no other satisfactory therapeutic options available. The primary intent of expanded access is to provide treatment for a patient’s disease or condition, rather than to collect data about the study drug.
Post-Trial access is the provision of an investigational drug for clinical trial participants after the trial ends. Participants in VBP15-002 and VPB15-003 were eligible to enroll in VBP15-LTE, a 24-month long-term extension clinical trial protocol. To accommodate post-trial access to vamorolone for participants after the completion of VBP15-LTE or VBP15-004, country-specific regulatory mechanisms had to be followed.
Expanded access in the EU refers to providing access to drugs that have been deemed safe and effective by a regulatory agency to a community (or country) – in addition to the trial participants—in which the clinical trials were conducted. This is also known as an “Early Access Program” or “EAP”. Therefore, when using the terms “expanded access” or “EAP”, it is very important to delineate whether the term is referring to provision of an investigational drug to individuals or a group of patients outside of a clinical trial (as in the US), or post-trial community (or country) access to a drug (“Early Access Program” in the EU) (see table for more details).
Access to Vamorolone for VISION-DMD participants completing the trial
Corticosteroids are prescribed in a wide variety of conditions, many of whom suffer from side effects of the drugs. Vamorolone holds potential in these other disorders, but to date is being developed for only Duchenne and Becker muscular dystrophies. At this point, access to vamorolone is limited to those DMD boys in clinical trials and those having completed clinical trials.
In the vamorolone Expanded Access protocol (VBP15-EAP), vamorolone is prescribed by a physician as part of his clinical care; the drug is provided at no cost to the patient. The parent must provide informed consent for their son to receive vamorolone. There are no “study visits”. The parent must provide informed consent for their son to receive vamorolone. While taking vamorolone, the patient continues to receive careful followup by his physicians (as he would if he were taking prednisone or deflazacort), and the prescribing physician agrees to report any side effects according to rules described in the protocol. This is important so that the Sponsor can react to and accurately report any serious side effects to the regulatory agencies. In Canada and Israel, there are currently no expanded access regulations as there are in the US. However, Health Canada and the Israeli Ministry of Health agreed to implement the same Expanded Access protocol in these countries.
In European countries and Australia, post-trial access is via different regulatory mechanisms. According to some country’s regulations, vamorolone did not qualify for a Compassionate Use Program due to the stage of development, or the limitation of access to those completing trials. In the UK, the drug is being provided under Named Patient Based Access. Australia also has a Named Patient Based Access program called the Special Access Scheme.
For more information on Compassionate Use and Early Access Programs in Europe, please visit the Eurordis website.
https://www.eurordis.org/content/links-national-authorities-websites
